
In other words, this chemical element is older than our planet. When a supernova explodes, it scatters iron, oxygen, and carbon atoms all over outer space, and by the force of gravity, it lands on other planets, asteroids, and meteors. How Was Iron Discovered?Įlement 26 is one of the elements that have been born in the Universe by the supernova explosions of mass stars. Being a member of the transition metals family of elements, this element has two valence electrons and numerous naturally occurring compounds. It’s also an excellent conductor of electricity and heat. Iron exists in 4 crystalline forms and possesses a ferromagnetic ability.
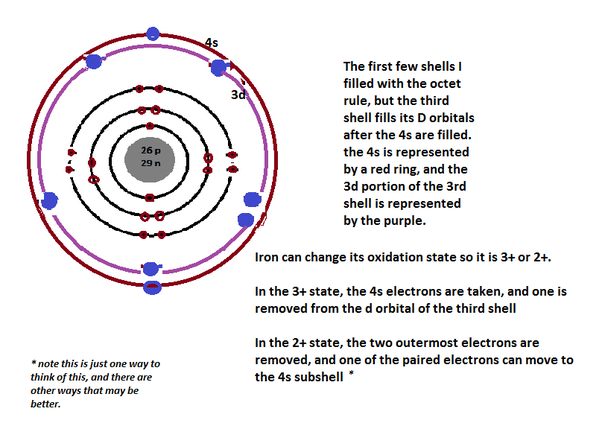
This member of the transition metals family of elements has an electronegativity of 1.8 according to Pauling, whereas the atomic radius according to van der Waals is 0.126 nm. It reaches its boiling point at 2861☌, 5182☏, 3134 K, while the melting point is achieved at 1538☌, 2800☏, 1811 K. With the periodic table symbol Fe, atomic number 26, atomic mass of 55.85 g.mol -1, and electron configuration 3d 6 4s 2, iron is a ductile, malleable, lustrous silver-gray metal. The energy of the third ionization: 2951 kJ.mol -1 The energy of the second ionization: 1556.5 kJ.mol -1 The energy of the first ionization: 761 kJ.mol -1 Half-life: From 1.89(49) milliseconds to 2.6 million yearsĮlectronegativity according to Pauling: 1.8 Physical state: Solid at room temperature The symbol in the periodic table of elements: FeĬolor: Iron black, dark gray, steel gray with metallic luster Fact Box Chemical and Physical Properties of Iron In this way, iron provides energy for the skeletal muscles, responsible for our physical movement. By binding to oxygen molecules, iron in our blood supports the diffusion of oxygen to all systems, tissues, and organs of the body. Its presence in our blood is critical for the proper functioning of the brain, muscles, and red blood cells. Iron is the most vitally important component of the hemoproteins hemoglobin and myoglobin. With an abundance of about 35% in both Earth’s inner and outer core, Fe is the fourth most abundant chemical element in Earth’s crust and the most common chemical element on Earth. Phenom., 1980, 21, 275.Iron is a chemical element with the atomic number 26 in the periodic table. Mårtensson, "Core-Level Binding Energies in Metals," J. Lide, (Ed.) in Chemical Rubber Company handbook of chemistry and physics, CRC Press, Boca Raton, Florida, USA, 81st edition, 2000. Ley, Eds., Photoemission in Solids I: General Principles (Springer-Verlag, Berlin) with additional corrections, 1978. Burr, "Reevaluation of X-Ray Atomic Energy Levels," Rev. They are tabulated elsewhere on the WWW (reference 4) and in paper form (reference 5). The data are adapted from references 1-3. I am grateful to Gwyn Williams (Jefferson Laboratory, Virginia, USA) who provided the electron binding energy data.

The binding energies are quoted relative to the vacuum level for rare gases and H 2, N 2, O 2, F 2, and Cl 2 molecules relative to the Fermi level for metals and relative to the top of the valence band for semiconductors. All values of electron binding energies are given in eV. 1967, 47, 1300.Įlectron binding energies Electron binding energies for iron. These effective nuclear charges, Z eff, are adapted from the following references:


 0 kommentar(er)
0 kommentar(er)
